
A short overview
Project B2 deals with the laboratory investigation of small molecular cations of astrochemical interest using cryogenic ion trapping machines. Building on the novel action spectroscopic methods developed in the last funding periods, one focus is the infrared and (sub)mm-wave spectroscopy of deuterated cations whose spectral fingerprints are not or insufficiently known. As the rotational spectroscopy of the primary deuterated ions (those created by gas-phase exchange collisions of H3+, CH3+ or C2H2+ with HD molecules) has been completed successfully, the project turns its attention to secondary deuterated ions. This spectroscopic knowledge is important for the interpretation of the deuterium fractionation observed during and after the initial cold phases of star formation. Additionally, small fundamental cations containing only hydrogen and helium (e.g. H2He+) will be investigated.
Understanding deuterium chemistry is a central tool in understanding the different phases of star formation. Therefore project B2 is right at the center topic of the SFB. The results of project B2 are of relevance for the other laboratory astrophysics project B3, since deuteration and understanding the formation of large carbon containing molecules are strongly related. Project A6 uses observations of the molecular deuteration as an important tool to study the earliest, cold phases of star formation and characterizes the physical and chemical conditions of the star forming clumps. In particular, the deuteration of the coldest objects is probed using H2D+ lines studied in this project. Furthermore, the results of the kinetics unraveled in project B2 are of importance for the chemical modeling in project C3.
Our Work
 Stars are formed from the collapsing dense and cold clumps contained in in interstellar clouds. In these cold environments, astronomers observe a wealth of molecules, in which a hydrogen atom (H) has been replaced by its heavier sibling deuterium (D). Astonishingly, the abundance of deuterated molecules in these cold clouds is by far more than one would expect by the environmental D/H ratio. This phenomenon is called deuterium enrichment (or deuterium fractionation). Deuterium fractionation is a central tool in understanding the different phases of star formation. Deuterated molecules are sometimes the only tracers for the coldest environments in space and thus are key to characterize star forming regions, especially prestellar cores and young protostellar envelopes.
Stars are formed from the collapsing dense and cold clumps contained in in interstellar clouds. In these cold environments, astronomers observe a wealth of molecules, in which a hydrogen atom (H) has been replaced by its heavier sibling deuterium (D). Astonishingly, the abundance of deuterated molecules in these cold clouds is by far more than one would expect by the environmental D/H ratio. This phenomenon is called deuterium enrichment (or deuterium fractionation). Deuterium fractionation is a central tool in understanding the different phases of star formation. Deuterated molecules are sometimes the only tracers for the coldest environments in space and thus are key to characterize star forming regions, especially prestellar cores and young protostellar envelopes.
Among the chemical processes occurring in cold clouds, the family of ion-molecule reactions H3+ +H2 (in all its isotopic variants) is of utmost importance, in particular for the deuteration processes. The sequential deuteration reactions
are the main initial drivers of deuteration in cold interstellar clouds and determine the chemistry in the last moments before star formation. Additionally, H 2 D+ and D2H+ are one of the few remaining molecular tracers, when all other heavier elements have been frozen out onto dust grains (H3+ does not have a dipole moment an d cannot be detected by its rotational motion).
It is the aim of this project to investigate these ion-molecule-reactions in quantum-mechanical detail, and supply the astrochemical community and observers with accurate rate coefficients as well as transition frequencies for deuterated species. Besides the important reactions system H3+ + H2, similar reactions involving CH 3 + and C 2 H2+will be examined.
Laboratory tools:
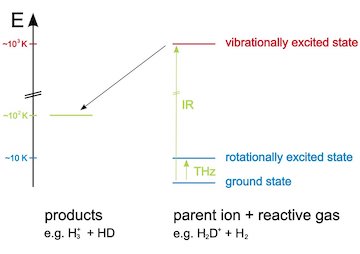
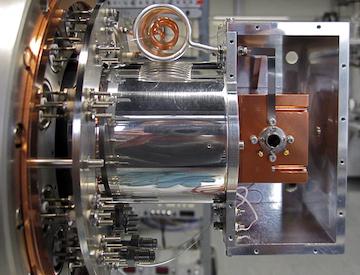 Ion-molecule reaction systems are best investigated in low temperature ion traps (22-pole traps). For the reaction system H 3 + + H2 the chemistry can be examined by changing the conditions, as the ambient temperature, the ortho/para-ratio of the H2 molec ules as well as the HD/H2-ratio of the reaction gas. For spectroscopic investigations, the method of laser induced reactions (LIR) can be used. It allows to obtain state-specific reaction rates and accurate ro-vibrational and rotational frequencies of the investigated deuterated ions. In detail, following laboratory tools are available for the investigation:
Ion-molecule reaction systems are best investigated in low temperature ion traps (22-pole traps). For the reaction system H 3 + + H2 the chemistry can be examined by changing the conditions, as the ambient temperature, the ortho/para-ratio of the H2 molec ules as well as the HD/H2-ratio of the reaction gas. For spectroscopic investigations, the method of laser induced reactions (LIR) can be used. It allows to obtain state-specific reaction rates and accurate ro-vibrational and rotational frequencies of the investigated deuterated ions. In detail, following laboratory tools are available for the investigation:
- 10K 22-pole ion trap machine. Allows to do spectroscopy and investigate ion-molecule reactions from 300K to the lowest temperature of 10K
- 4K 22-pole ion trap machine. As all gases are frozen out at 4K (except Helium), this machine is used for high-resolution spectroscopy in combination wit a frequency comb (see below)
- para-H2 generator. Using a paramagnetic catalyst at low temperature, commercial hydrogen gas (normal-hydrogen) can be converted into high-purity para-hydrogen samples. A Raman-spectrometer is available to control the purity.
- frequency comb. For high-resolution spectroscopy of cold (4K) ions, the calibration of the frequency axis is very important. This is achieved with a frequency comb locked to a rubidium atomic clock. For mid-IR spectroscopy, this system enables relative accuracies better than 10-9.
Goals and workplan
 Develop reliable methods to produce and characterize high-purity p-H2 samples.
Develop reliable methods to produce and characterize high-purity p-H2 samples.- For a H3+ like sample stored in H2 gas (containing impurities of HD), determine the H2D+/H3+ and the D 2 H+/H3+ ratio as a function of the trap temperature, the HD/H2 ratio and the o/p-ratio of H2.
- Use a microcanonical model to compare the above experimental results. Further develop the model to include different reaction mechanisms.
- Determine the o/p-ratio of H3+ embedded in a cold cloud of o/p-H2.
- The ultimate goal for the H3+ system (and its deuterated siblings) is to obtain state-to-state rate coefficients which are finally needed for a proper comparison of theory and experiment as well as for the proper modelling of the deuterium chemistry.
- Determine accurate transition frequencies for important deuterated ions, in particular CH 2 D+ and C2HD+.
Selected Publications
-
2022
- P.C. Schmid, O. Asvany, T. Salomon, S. Thorwirth, S. Schlemmer, Leak-Out Spectroscopy, A Universal Method of Action Spectroscopy in Cold Ion Traps, The Journal of Physical Chemistry A, 126 (43), 8111-8117 (2022)
- K.M.T. Yamada, O. Asvany, S. Schlemmer, K-type doubling in the rotational spectrum of CH3+–He: A group theoretical explanation, Journal of Molecular Spectroscopy, Volume 388, 111670 (2022)
- T. Salomon, S. Brackertz, O. Asvany, I. Savić,. S. Schlemmer, "The He–H3+-complex. II. Infrared predissociation spectrum and energy term diagram", J. Chem. Phys. 156, 144308 (2022)
- S. Thorwirth, O. Asvany, M.E. Harding, P. Jusko, M.C. McCarthy, S. Brünken, S. Schlemmer, Infrared action spectroscopy of fundamental nitrilium ions: Protonated vinyl- and ethyl cyanide, Journal of Molecular Spectroscopy, Volume 386, 111615 (2022)
- P.C. Schmid, S. Thorwirth E.C. Endres, M. Töpfer, A. Sánchez-Monge, A. Schwörer, P. Schilke, S. Schlemmer, O. Asvany, Rotational Rest Frequencies and First Astronomical Search of Protonated Methylamine, Frontiers in Astronomy and Space Sciences, 8, (2022)
-
2021
- O.Asvany, S. Schlemmer, Rotational action spectroscopy of trapped molecular ions, Physical Chemistry Chemical Physics, vol. 23, issue 47, pp. 26602-26622 (2021)
- O. Asvany, C.R. Markus, A. Roucou, S. Schlemmer, S. Thorwirth, C. Lauzin, The fundamental rotational transition of NO+, Journal of Molecular Spectroscopy, Volume 378, 111447 (2021)
- T. Salomon, J.L. Doménech, P.C. Schmid, E.A. Michael, S.Schlemmer, O.Asvany, Rovibrational spectroscopy of the CH+-He and CH+-He4 complexes, Journal of Molecular Spectroscopy, Volume 377, 111421 (2021)
- O. Asvany, S. Schlemmer, A. van der Avoird, T. Szidarovszky, A.G. Császár, Vibrational spectroscopy of H2He+ and D2He+, Journal of Molecular Spectroscopy, Volume 377, 111423 (2021)
- O. Asvany, C. R. Markus, K. Nagamori, H. Kohguchi, J. Furuta, K. Kobayashi, S. Schlemmer, S. Thorwirth, Pure Rotational Spectrum of CCl+, The Astrophysical Journal, Volume 910, 1 (2021)
-
2020
- O. Asvany, C. R. Markus, T. Salomon, P. C. Schmid, S. Banhatti, S. Brünken, F. Lipparini, J. Gauss, S. Schlemmer, "High-resolution rovibrational spectroscopy of c-C3H2+ : The ν7 C–H antisymmetric stretching band", J. Mol. Struct., 1214, Art. No. 128023 (2020)
- C. R. Markus, O. Asvany, T. Salomon, P. C. Schmid, S. Brünken, F. Lipparini, J. Gauss, and S. Schlemmer, "Vibrational Excitation Hindering an Ion-Molecule Reaction: The c−C3H2+−H2 Collision Complex", Phys. Rev. Lett., 124, Art. No. 233401 (2020)
- B. A. McGuire, O. Asvany, S. Brünken, S. Schlemmer, "Laboratory spectroscopy techniques to enable observations of interstellar ion chemistry", Nat. Rev. Phys., 2, 402–410 (2020)
- J.L. Doménech, O. Asvany, C. R. Markus, S. Schlemmer and S. Thorwirth, "High-resolution infrared action spectroscopy of the fundamental vibrational band of CN+", J. Mol. Spectrosc., 374, 111375 (2020)
- S. Thorwirth, M. E. Harding, O. Asvany, S. Brünken, P. Jusko, K. L. K. Lee, T. Salomon, M. C. McCarthy, and S. Schlemmer, "Descendant of the X-ogen carrier and a "mass of 69": Infrared action spectroscopic detection of HC3O+ and HC3S+", Mol. Phys., 118, e1776409 (2020)
-
2019
- PT. Salomon, M. Töpfer, P. Schreier, S. Schlemmer, H. Kohguchi, L. Surin, and O. Asvany, "Double resonance rotational spectroscopy of He–HCO+", Phys. Chem. Chem. Phys., 21, 3440–3445 (2019)
- M. Töpfer, P. C. Schmid, H. Kohguchi, K. M. T. Yamada, S. Schlemmer, O. Asvany, "Infrared photodissociation of cold CH3+–He2 complexes", Mol. Phys., 117, 1481–1485 (2019)
- A. G. Császár, T. Szidarovszky, O. Asvany, S. Schlemmer, "Fingerprints of microscopic superfluidity in HHen+ clusters", Mol. Phys., 117, 1559–1583 (2019)
- S. Thorwirth, P. Schreier, T. Salomon, S. Schlemmer, and O. Asvany, "Pure rotational spectrum of CN+", Astrophys. J. Lett., 882, L6 (2019)
- P. Jusko, S. Brünken, O. Asvany, S. Thorwirth, A. Stoffels, L. van der Meer, G. Berden, B. Redlich, J. Oomens, and S. Schlemmer, "The FELion cryogenic ion trap beam line at the FELIX free-electron laser laboratory: Infrared signatures of primary alcohol cations", Faraday Discuss., 217, 172–202 (2019)
- O. Asvany, S. Schlemmer, T. Szidarovszky, A. G. Császár, "Infrared Signatures of the HHen+ and DHen+ (n = 3–6) Complexes", J. Phys. Chem. Lett., 10, 5325–5330 (2019)
- C. R. Markus, S. Thorwirth, O. Asvany, S. Schlemmer, "High-resolution double resonance action spectroscopy in ion traps: vibrational and rotational fingerprints of CH2NH2+", Phys. Chem. Chem. Phys., 21, 26406–26412 (2019)
-
2018
- Asvany, O., Thorwirth, S., Redlich, B., and Schlemmer, S., Spectroscopy of the low-frequency vibrational modes of CH3+, J. Mol. Spectrosc., 2018, 347, 1
- Domenech, J. L., Jusko, P., Schlemmer, S., and Asvany, O., The first laboratory detection of Vibration-Rotation Transitions of CH+ and 13CH+ and Improved Measurement of their Rotational Transition Frequencies, Astrophys. J., 2018, 857, 61
-
2017
- Domenech, J. L., Schlemmer, S., and Asvany, O., Accurate Frequency Determination of Vibration-Rotation and Rotational Transitions of SiH+, Astrophys. J., 2017, 849, 60
- Jusko, P. et al., Double resonance rotational action spectroscopy of cold H2D+ and D2H+, J. Mol. Spectrosc., 2017, 332, 33
- Jusko, P. et al., High-resolution vibrational and rotational spectroscopy of CD2H+ in a cryogenic ion trap, J. Mol. Spectrosc., 2017, 332, 59
- Harju, J. et al., Detection of Interstellar Ortho-D2H+ with SOFIA, Astrophys. J., 2017, 840, 63
- Fanghänel, S., Schlemmer, S., and Asvany, O., Optimization of RF multipole ion trap geometries, J. Mol. Spectrosc., 2017, 332, 124
-
2016
- Jusko, P., Konietzko, C., Schlemmer, S., and Asvany, O., Frequency comb assisted measurement of fundamental transitions of cold H3+, H2D+ and D2H+, J. Mol. Spectrosc., 2016, 319, 55
- Töpfer, M., Jusko, P., Schlemmer, S., and Asvany, O., Double resonance rotational spectroscopy of CH2D+, Astron. Astrophys., 2016, 593, L11
-
2015
- Savic, I. et al, Controlled synthesis and analysis of He-H3+ in a 3.7 K ion trap, Mol. Phys., 2015, 113, 15
-
2014
- Asvany, O., Brünken, S., Kluge, L., and Schlemmer, S., COLTRAP: a 22-pole trapping machine for spectroscopy at 4 K. Appl. Phys. B, 2014, 114, 203
- Brünken, S., Kluge, L., Stoffels, A., et al., Laboratory rotational spectrum of l-C3H+ and confirmation of its astronomical detection. Astrophys. J. Lett., 2014, 783, L4 (co-authors: Asvany, O., Schlemmer, S.)
-
2013
- Gärtner, S., Krieg, J., Kleman, A., et al., High-resolution spectroscopy of CH2D+ in a cold 22-pole ion trap. J. Phys. Chem. A, 2013, 117, 9975 (co-authors: Asvany, O., Schlemmer, S.)
-
2012
- Asvany, O., Krieg, J., and Schlemmer, S., Frequency comb assisted mid-infrared spectroscopy of cold molecular ions. Rev. Sci. Instr., 2012, 83, 093110
- Kluge, L., Gärtner, S., Brünken, S., et al., Transfer of a proton between H2 and O2 . Phil. Trans. Royal Soc. A, 2012, 379, 5041 (co-authors: Asvany, O., Schlemmer, S.)
- Grussie, F., Berg, M.H., Crabtree, K.N. et al., The low temperature nuclear spin equilibrium of H3+ in collisions with H2. Astrophys. J., 2012, 759, 21 (co-authors: Gärtner, S., Schlemmer, S.)
-
2010
- Asvany, O., Bielau, F., Moratschke, D., et al., A new design of a cryogenic linear radio frequency multipole trap. Rev. Sci. Instr., 2010, 81, 076102 (co-authors: Schlemmer, S.)
-
2008
- Asvany, O., Ricken, O., Müller, H.S.P., et al., High-resolution rotational spectroscopy in a cold ion trap: H2D+ and D2H+. Phys. Rev. Lett., 2008, 100, 233004 (co-authors: Schlemmer, S.)
Our Team
Dr. Oskar Asvany (PI, PH1), Thomas Salomon (PH1), Matthias Töpfer (PH1)
Former members
Alexander Stoffels (PH1), Sven Fanghänel (PH1), Lars Kluge (PH1), Sabrina Gärtner (PH1), Philip Schreier (PH1)
